Oral Homeostasis
The majority of oral microbiota species exist within a complex multispecies structure termed the oral biofilm (dental plaque). The development of oral biofilms is initiated by adherence of pioneer species to salivary proteins and glycoproteins adsorbed onto tooth enamel. In healthy individuals, the oral biofilm is dominated by commensal bacteria that maintain homeostasis through intricate interactions with host immunity and other microorganisms.
Early colonisers of the mouth are commensal Streptococci spp. including S. mitis, S. sanguinis and S. gordonii. These early colonisers are able to bind to the tooth surface and prevent colonisation of other bacteria through production of bacteriocins, H2O2 and alkali production. Dominance of commensal Streptococci is associated with good oral health.
Late colonisers, including Gram-negative (i.e. Veillonella spp.) and Gram-positive species (i.e. Actinomyces spp.) build up on the initial Streptococcal biofilm via cell-cell interactions. This series of co-aggregation and co-adhesion events leads to the formation of a mature biofilm, a relatively stable community where all species exist in homeostasis.
Oral Dysbiosis
The disruption of homeostasis, termed dysbiosis, has been linked to a multitude of conditions including dental caries, gingivitis, increased risk of oral cancer, and most commonly, periodontitis.
Periodontitis is a chronic inflammatory disease of the periodontium – the tissues that surround and support the teeth. It affects 10–15% of adults and is the most common cause of tooth loss worldwide. Susceptibility is influenced by host genotype, stress, diet and associated behaviour, including smoking. It is caused by a synergistic and dysbiotic polymicrobial biofilm community, with keystone pathogens such as Porphyromonas gingivalis initiating the disruption of tissue homeostasis.
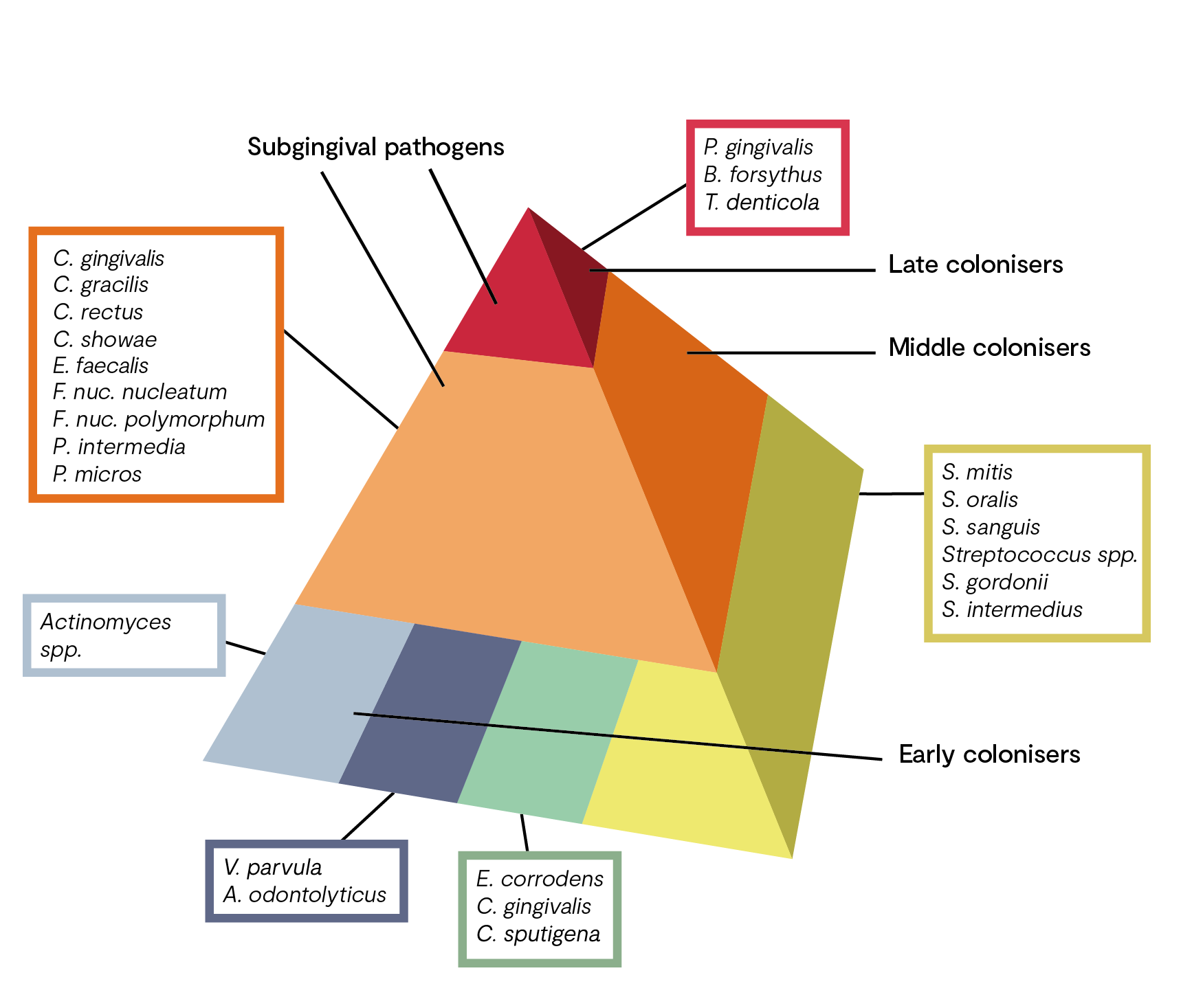
The process towards dysbiosis is often depicted in the research as a pyramid with ‘complexes’ of bacteria species (see figure 1). Early colonisers include members of the yellow, green, and purple complexes (oral health). The orange complex bacteria generally appear after the early colonisers and include many putative periodontal pathogens, such as Fusobacterium nucleatum.
The orange complex includes bacteria which, as “bridge species”, form a link between the early colonisers and the highly pathogenic bacteria of the red complex. The pathogenic potential of orange complex bacteria is significantly increased due to the production of various toxins and enzymes.
Occurrence of bacteria of the red complex is characteristic of the final colonisation phase, which culminates in the development of a structured, stable bacterial community (climax community) and includes putative periodontal pathogens. Colonisation with these bacteria, which are significantly involved in the destruction of the periodontium, builds on the presence of the aforementioned less pathogenic species.
In a dysbiotic oral microbiome, the host immune system is chronically activated, predominantly mediated by IL-1, IL-8, TNF-α, prostaglandins and matrix metalloproteinases (MMPs). These mediators affect functions and activities of leukocytes, osteoblasts and osteoclasts, leading to the characteristic destruction of the periodontal ligament, connective tissue and alveolar bone seen in chronic periodontitis.
What You Can Do: Clinical Tools
Much of this damage is reversible if pathogenic communities are removed, and homeostasis is restored; therefore, analysis and monitoring of the oral microbiota is important for health and can be used to inform protocols and lifestyle factors.
Your clinical tool: Oral EcologiX™ | Oral Health & Microbiome Profile





