An Introduction
Communication between the gut and the brain is transmitted via neural, hormonal, metabolic and immunological pathways. The gut is surrounded by a fully autonomous nervous system – the enteric nervous system (ENS) – which has a direct link to the central nervous system via the vagus nerve, which transmits signals from the brain to the gut and vice versa (1).
Our gut microbiota is important for maintaining healthy mucosal surfaces, and thus are key players in the gut-brain axis via their interaction with the ENS. They also modulate expression of neurotransmitters, hormones, and immunological mediators.
The Role of the Intestinal Barrier
The intestinal barrier, along with the microbiota, play a key role in homeostasis and in the defence against pathogens. If the intestinal barrier is impaired, a greater burden of immunomodulating substances such as lipopolysaccharides (LPS), can enter the system. This can lead to low-grade intestinal inflammation, which has been directly associated with depression, anxiety, panic attacks and other stress-related conditions, (2) suggesting that depression could be directly linked to a “leaky gut” (3). See figure 1:
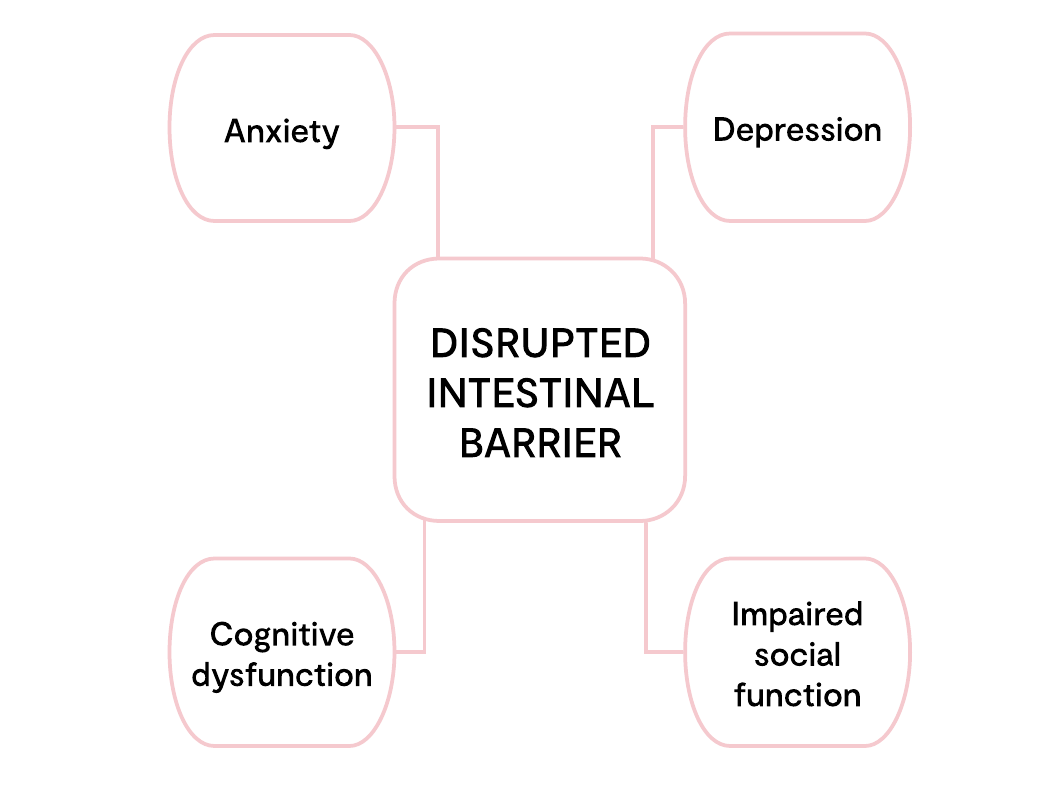
Figure 1 adapted from Kelly et al (2015).
Potential neuropsychiatric consequences of a dysregulated intestinal barrier. Activation of brain-gut microbiota axis signalling pathways via a compromised intestinal barrier with potential effects on mood, anxiety, cognition and social interaction.
Lipopolysaccharides: An Introduction
LPS is a component of the outer membrane of Gram-negative bacteria. There is about 1g of LPS in the human intestinal tract, and in a healthy state there is only a small amount of LPS present in plasma due to the effective barrier of the intestinal epithelial wall (4).
LPS induces intestinal hyper-permeability directly by down regulating the expression of occludin and ZO-1 mRNAs, while up regulating Cdkn1a. Thus, LPS is able to significantly increase paracellular permeability and epithelial barrier damage, adversely affecting the functionality of the intestinal epithelial barrier (5).
LPS is important for regulating the neural system. It increases the activity of the amygdala which is responsible for regulating emotion, affecting the physical activity of the brain and regulating production of neuropeptides (6).
LPS-induced inflammation can increase indoleamine 2,3 dioxygenase (IDO), which pushes tryptophan through the kynurenine pathway, and correlates positively with symptoms of depression (4).
LPS can directly damage the blood–brain barrier, consequently allowing substances such as environmental toxins, pathogens and LPS itself, to enter, inducing a neuro-inflammatory response (4).




