Oestrogen Bioactives | Research
Certain plants and nutrients may encourage the production of the enzymes involved in oestrogen metabolism and may support a healthy oestrobolome.
Many of the enzymes required for oestrogen biotransformation are also used in the clearance of xenoestrogens and other toxins. This week we will focus on two bioactives: broccoli seed extract and myrosinase.
Broccoli seed extract (TrueBroc®)
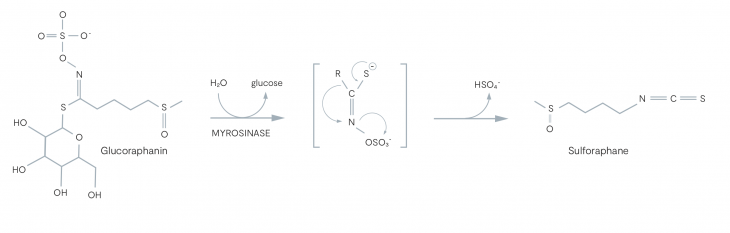
TrueBroc® is a water extract of sprouted broccoli seeds, which have an especially high glucoraphanin content. Glucoraphanins are further metabolised into the well-researched, sulforaphane.
Glucoraphanin and its metabolites are known for their ability to induce xenobiotic-metabolising enzymes that protect DNA from damage (1) and they are frequently researched for their antioxidant, chemoprotective, anti-inflammatory, neuroprotective and anti-cancer effects, due to their ability to induce detoxification enzymes (2-4).
Indeed, multiple population group studies show a correlation with cruciferous vegetable intake and lower rates of breast and prostatic cancers, amongst others (5).
Mustard seed myrosinase
The pathway for sulforaphane production is dependent on an enzyme known as myrosinase, which is stored within plant cells. When plant cells are damaged by cutting or chewing, myrosinase is released and comes into contact with glucoraphanin, hydrolysing them into their more active versions, such as sulforaphane.
Studies have shown that combining an endogenous source of myrosinase with broccoli seed extract can increase the production and bioavailability of sulforaphane by 3-4 fold (6).
Sulforaphane
Sulforaphane has multiple mechanisms of action. It possesses anti-carcinogenic activity via drug-metabolising enzymes, inducing cell cycle arrest and apoptosis, preventing inhibition of angiogenesis and metastasis, and histone acetylation (7,8). It also shows antioxidant, anti-inflammatory and immunomodulatory activities, through increased production of Nrf2 (7,8).
Sulforaphane has been shown to induce detoxification enzymes that alter oestrogen metabolism and protect against oestrogen-mediated DNA damage in postmenopausal women (9). Cultures of human cell lines exposed to sulforaphane showed CYP1B1 protein expression was significantly inhibited by approximately 50% with a 2.5-fold significant increase in COMT protein levels. Both of these factors favour the 2-methoxyestrone production over the 4-hydroxyestrone (4-OH), which is the form more likely to create quinolated conjugates.
Indeed, sulforaphane has been shown to reduce the growth of four different breast cancer cell lines in vitro (10). In cultured prostate cells, sulforaphane and broccoli seed extract potently induced quinone reductase activity and increased intracellular glutathione levels. This prevented the generation of highly reactive semiquinones that can go on to cause DNA oxidative damage (11).




