Phase one detoxification – cytochrome enzyme system
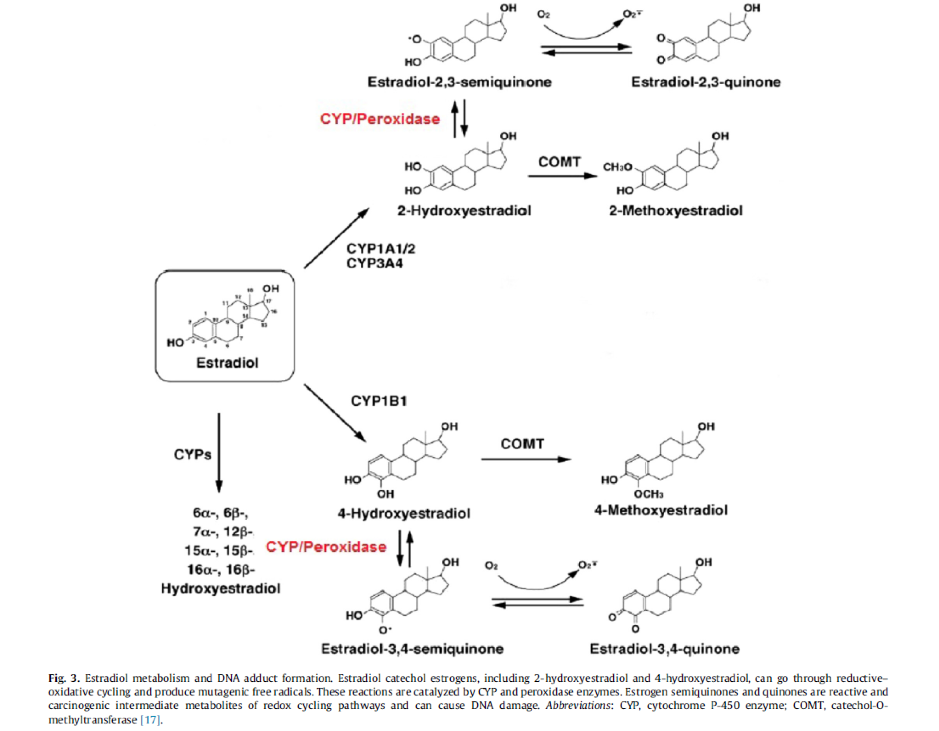
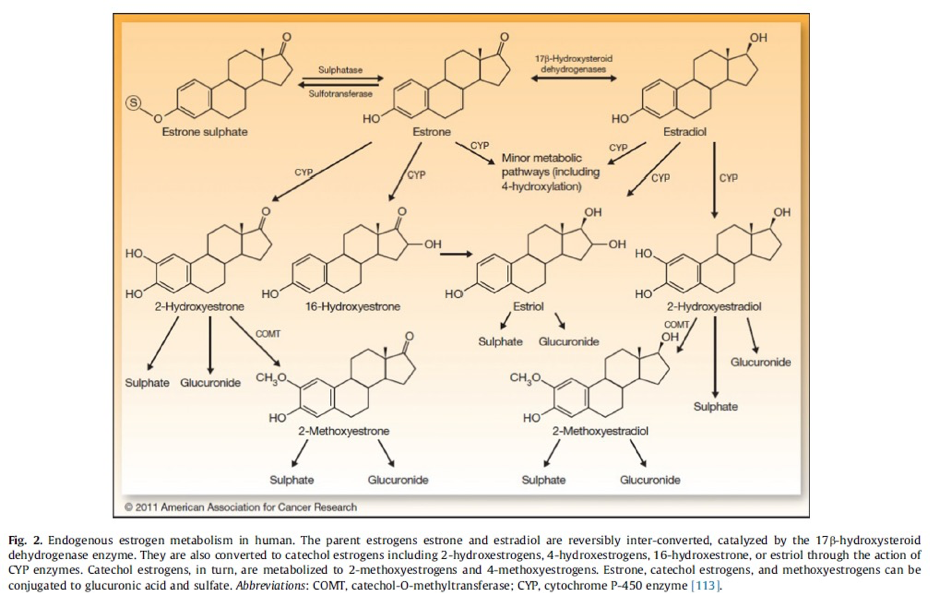
The cytochrome (CYP) family of detoxification enzymes are responsible for the first stages of oestrogen biotransformation, which predominantly occurs in the liver tissue. Oestrone and oestradiol can either utilise the enzymes CYP 1A1, CYP 1B1 or CYP3A4, dependant on what enzyme is more available at the time. Oestrogen undergoes an addition of a hydroxyl group (-OH) to become:
- 2-hydroxyestrone and 2- hydroxyestradiol –the main metabolites from the route of CYP1A1
- 4- hydroxyestrone and 4-hydroxyestradiol – mainly from interaction with CYP1B1
- 16α- hydroxyestrone – mainly from interaction with CYP3A4
- Oestriol is made mainly by the hydroxylation of oestradiol or of 16α- hydroxyestrone (1–3)
Each of these metabolites are highly reactive oxidative intermediates. They need to go through the rest of the biotransformation process to be able to be ready for excretion, and at this stage they can still act upon oestrogen receptors, albeit in a weaker form than their predecessors. 16α- hydroxyestrone has the strongest binding effects to the oestrogen receptors and possesses high proliferative effects. Higher urinary levels of 16α- hydroxyestrone have been found to been related to post- menopausal breast cancer (4).
4- hydroxyestrone also may possess a cancer promoting risk if it is not cleared through the second stage of biotransformation. It can in turn head down a different pathway to become the free radical, 3,4-quinone which possesses the ability to react with DNA and cause damage (1,4).
2-hydroxyestrone has the weakest binding potential to the oestrogen receptor and has also shown to have anti-proliferative effects on cancer cell lines, therefore it is thought to be the most beneficial of the metabolites (5).
Encouraging the availability of the CYP 1A1 enzyme and reducing the production of other metabolites in itself may help to reduce the toxic effects of a high circulating oestrogen load. Figure 1: (3)

Phase 2 detoxification – methylation, sulphation and glucuronidation
Once hydroxylated, the catechol oestrogens (2-OH and 4- OH) undergo the addition of a methyl group by the enzyme catechol-O- methyltransferase (COMT). This yields the metabolites 2-methoxyestrone, 4-methoxyestrone, 2-methoxyestradiol and 4-methoxyestradiol. These by products are considered safer and less reactive than their predecessors as they no longer will react with DNA.
Methylation of the catechol oestrogens also prevents them biotransformation to quinone-DNA adducts and development of reactive oxygen species (ROS) capable of damaging cellular macromolecules such as DNA, lipids, and proteins. Higher 2-hydroxylation of parent oestrogens is associated with lower risk of breast cancer, and low methylation 4-hydroxylation pathway catechols is associated with higher risk of postmenopausal breast cancer (5).
In addition to methylation, parent and catechol oestrogens are also conjugated with either glucuronic acid and sulfate by hepatic phase II enzymes including UDP-glucuronosyltransferases and sulfotransferases. Conjugation is the process by which hormones become more water soluble and are excreted in the urine or faeces or turn into a more lipophilic moiety with elevated half-lives (1). Conjugated metabolites are normally water soluble and excreted via the kidneys or bile. Sulphated oestrogen metabolites can be directly converted back to oestradiol so add to the circulating oestrogen load. Gluronidated oestrogens can be deconjugated back into free oestrogens by the oestrobolome, noted below. The enzyme β-glucuronidase, which is found in the breast gland, is produced by bacterial synthesis in the gut microbiome. Abnormally high level of microbial β-glucuronidase in the intestine can deconjugate gluronidated oestrogens, allowing them to be reabsorbed in the enterohepatic circulation and contributing to the overall oestrogen load.

Phase 3 metabolism – anti-porter activity
The final stage of oestrogen metabolism is removing the metabolite from the cell, so it can then be excreted either through the bile or the urine. Movement of the metabolites out of the cell at this stage is due to the activity of anti-porters, which can be upregulated by certain substances.
The oestrobolome
Gluronidated oestrogens can be deconjugated back into free oestrogens by the enzyme β-glucuronidase. β-glucuronidase is an enzyme that occurs in the body, for example it is found in high amounts in the breast tissue, but it can also be produced by bacterial synthesis in the gut microbiome. High levels of microbial beta-glucuronidase in the intestine can deconjugate gluronidated oestrogens which have been excreted in bile into the intestine, allowing them to be reabsorbed in the enterohepatic circulation and contributing to the overall oestrogen load.
Genetic variances
Genetic polymorphisms in genes encoding enzymes involved in oestrogen metabolism pathways and the genes encoding the oestrogen receptors (ERs) are associated with breast cancer risk. Polymorphic variations in genes encoding COMT, CYP1A1, CYP1B1, oestrogen receptor alpha (ERa), oestrogen receptor beta (ERb), CYP17A1, and CYP19A1 are all suspected to play a role in larger development of diseases.