Menopause refers to the time marking the end of a woman’s menstrual cycle, occurring when the ovaries cease releasing eggs resulting in declining levels of ovarian hormones – predominately β-estradiol, but also oestrone, progesterone and testosterone.
The average age of menopause in the UK is 51 for women of white and asian ethnicity and around 2 years earlier for women of black ethnicity, with anytime after 45 considered normal.
Menopause is a natural part of ageing; however, in some women, early menopause can occur. For 4.9-9.4% this can be between 40-45 and premature menopause (referred to as premature ovarian insufficiency – POI) can occur in 1%–8.6% of women under 40¹⁴.
Perimenopause is the term given to the transition period leading up to menopause. This is the beginning of menstrual irregularities (initially shorter than longer cycles) and when symptoms of female sex hormone fluctuations begin. The average onset of perimenopause is 47.5 years, although these figures again vary by race and ethnicity.
This life stage is associated with a constellation of symptoms and conditions that fall into three distinct areas:-
- Metabolic derangement; redistribution of adipose tissue to predominantly visceral. , This can increase the occurrence of a number of metabolic disorders, including fully manifested metabolic syndrome, and enhances the development of atherosclerosis, insulin resistance, increases in total cholesterol, LDL-C and ApoB1 and cardiovascular risk¹¹.
- Vasomotor symptoms (VSMs) (hot flushes and night sweats), sleep disturbances and mood changes; depression and low libido.
- Genitourinary symptoms; vulvovaginal atrophy (VVA), dyspareunia, dysuria, and recurrent urinary tract infections (UTIs).
This article focuses on the interplay between the different microbiomes at this life stage with their associated symptoms and conditions. Gaining a deeper appreciation of the gut microbiota-brain axis, gut-vaginal axis and gut-vaginal-bladder axis all of which provide bidirectional interactions involving metabolic, neuroendocrine, and immune pathways provides an opportunity for an integrative approach and targeted therapeutic interventions to assist in the amelioration in the three categories described previously.
The gut microbiome oestrogen and metabolic derangement
The gut microbiome, which consists of the microbes in the intestinal tract and their metabolites, plays an important role in overall health. It has been shown that the drop in oestrogen levels in menopause can cause changes to the gut microbiota, to a pattern that is associated with central adiposity, decreased metabolic rate, and insulin resistance².
A healthy and diverse microbiome comprises primarily 4 phyla: Bacteroidetes, Firmicutes, Actinobacteria and Proteobacteria, with over 90% of species falling within the Bacteroidetes & Firmicutes phyla. A reduction to this diversity, termed dysbiosis, results from an abnormal balance between commensals – a higher Firmicutes to Bacteroidetes ratio and the presence of more pathogenic bacterial species. Many studies have suggested a direct relationship between dysbiosis, and inflammatory milieu and metabolic derangement⁷.
Studies found that in women postmenopause, as a direct result of reduced circulating oestrogen the relative abundance of Firmicutes increased and Bacteroidetes decreased, resulting in a significantly higher Firmicutes to Bacteroidetes ratio. This suggests that menopausal changes may induce gut dysbiosis, leading to a higher risk to develop health-related problems including weight gain¹⁸.
Declining levels of progesterone and oestrogen in late-stage perimenopause and menopause has also been shown to cause the downregulation of epithelial junction proteins, gut barrier dysfunction, and an increase in gut permeability. This, in turn, permits the translocation of LPS microbes into the subepithelial space, triggering immune cells to produce proinflammatory cytokines which drive insulin resistance and weight gain⁴.
Interestingly, Zhou et al. (2019) demonstrated¹⁹ in their in vitro study that progesterone decreased the inflammatory protein, NF-κB, activation in response to exposure to bacterial lipopolysaccharide exposure (LPS), and further decreased permeability. These results suggest that the increased levels of progesterone may contribute to decreases in mucosal permeability and consequently microbial translocation and systemic inflammation.
Therefore it can be concluded that there is an even greater need at this life stage to focus on lifestyle counselling clients to remove environmental and dietary factors which are known to contribute to further a dysbiotic and inflammatory state.
The gut-vagina-bladder-oestrogen axis
A variety of tissues express oestrogen receptors including; intestine, brain, bone and adipose tissue. While the sex hormone milieu appears to impact the gut microbiome and barrier integrity in one direction of the axis, the gut microbiota also impacts the levels of free circulating hormone levels, in a bidirectional relationship, demonstrating the influence the gut microbiome has on oestrogen-related health outcomes.
The vaginal microbiome is a dynamic ecosystem that varies substantially throughout a woman’s lifespan. In healthy premenopausal women, physiological levels of circulating oestrogen stimulates glycogen accumulation in the vaginal epithelium, promoting dominance by one or a few Lactobacillus species (L. crispatus, Lactobacillus iners, Lactobacillus jensenii or Lactobacillus gasseri). Fermentation of glycogen by Lactobacillus yields lactic acid, which reduces vaginal pH to 3.5–4.5. The acidic vaginal microenvironment in turn inhibits colonisation by bacterial, viral, fungal and protozoan pathogens.
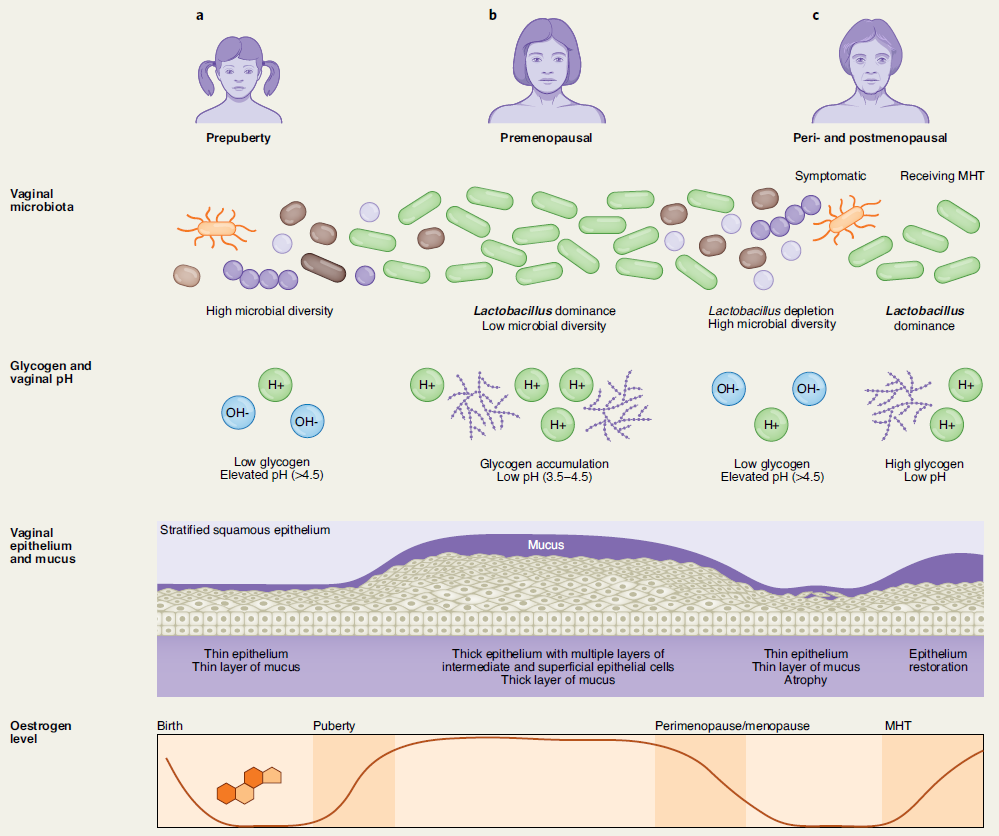
Declining circulating oestrogen levels from >450 pmol/L to <36 pmol/L ml. in the later stage of peri- and postmenopausal women results in reduced glycogen in the vaginal epithelium and consequently a decrease in Lactobacillus abundance. As a direct result, vaginal epithelia that are now less acidified consequently become colonised by anaerobic bacteria such as Gardnerella, Prevotella, Anaerococcus, Peptoniphilus and Peptostreptococcus. As well populations of pathobionts Escherichia, Enterococcus, Streptococcus, Corynebacterium and Staphylococcus in postmenopausal women have also been frequently reported (8-9).
This decrease in lactobacillus abundance consequently results in elevated vaginal pH leading to higher microbial diversity together with concurrent thinning of the vaginal epithelium atrophy, dyspareunia, dysuria and other genitourinary symptoms of menopause (GSM) in part from the changes in the local microenvironment.
GSM affects approximately 50% of postmenopausal women and can significantly impair women’s health, sexual function and quality of life. It has been postulated that the vaginal microbiome closely relates to vaginal health and GSM. A 2013 cross-sectional study (n = 87) showed that women with signs of mild or moderate VVA had greater odds to have highly diverse microbiota depleted of Lactobacilli than microbiota dominated by Lactobacillus crispatus when compared to women with no VVA⁸.
The interconnectivity of microbiota within the urogenital tract
Vaginal Lactobacillus species have also been hypothesised to play a protective role within the urinary tract. Dysuria and recurrent urinary tract infections (UTIs) are common symptoms in postmenopausal women. A 2021 study identified anaerobic bacteria (for example, Prevotella) to be associated with recurrent UTIs treated with antibiotics. Overall, restoration of vaginal Lactobacilli might help alleviate some urinary symptoms and protect against UTIs.
Intriguingly, a study of postmenopausal women (n = 62) with overactive bladder symptoms also revealed that increased levels of Lactobacillus in the bladder were associated with modest improvement in urgency and incontinence symptoms. From the opposite direction, the urinary tract might serve as a reservoir of vaginal Lactobacillus species and aid with the re-colonization of the reproductive tract after dysbiosis occurs.
Lactobacillus iners lacks the ability to synthesise D-lactic acid which is more protective against vaginal dysbiosis than L-lactic acid. D- Lactic acid levels are highest when L. crispatus is the dominant species and lowest when L. iners, Gardnerella or Streptococcus predominate; and this partly accounts for the higher protection against urogenital infections.
As such, the use of a Vaginal Health and Microbiome Profile, for analysis of a client’s personal vaginal microbiota composition using PCR (qRT-PCR) and enzyme-linked immunosorbent assays (ELISA), provides an accurate, reliable and quantifiable measurement of each client’s microbiome for a more bespoke intervention.
The gut microbiota can also impact microbial communities in the vagina via the oestrobolome. The oestrobolome is defined as the collection of enteric microorganisms (and their genes) capable of metabolising oestrogens⁹. Some enteric bacteria can produce enzymes, such as β-glucuronidases and sulfatases to reactivate previously conjugated oestrogens into their active forms, which consequently decrease their excretion and stimulate their reabsorption into circulation¹⁷.
Endogenously produced oestrogens, namely oestradiol (predominant in pre-menopause), and oestrone (predominant in post-menopause) are metabolized in the liver through first-pass hydroxylation and subsequent conjugation with glucuronide or sulfate groups. The glucuronide or sulphate conjugation in the liver allows for the biliary excretion of oestrogens via the intestinal tract. However, some gut microbiota, collectively termed the oestrobolome possess the ability to deconjugate these oestrogens, thereby releasing them for reabsorption into the enterohepatic circulation. It is these “active” de-conjugated and unbound oestrogens that enter the bloodstream and subsequently act on oestrogen receptors alpha (ERα) & beta (ERβ) present in multiple tissues.
Many gut species across major gut phyla, especially Firmicutes and Bacteroidetes, possess β-glucuronidase and sulfatase enzymes, acting in the deconjugation of glucuronide or sulphate groups, respectively. Escherichia coli, Bacteroides spp. and Clostridium perfringens dominate the species with this capacity.
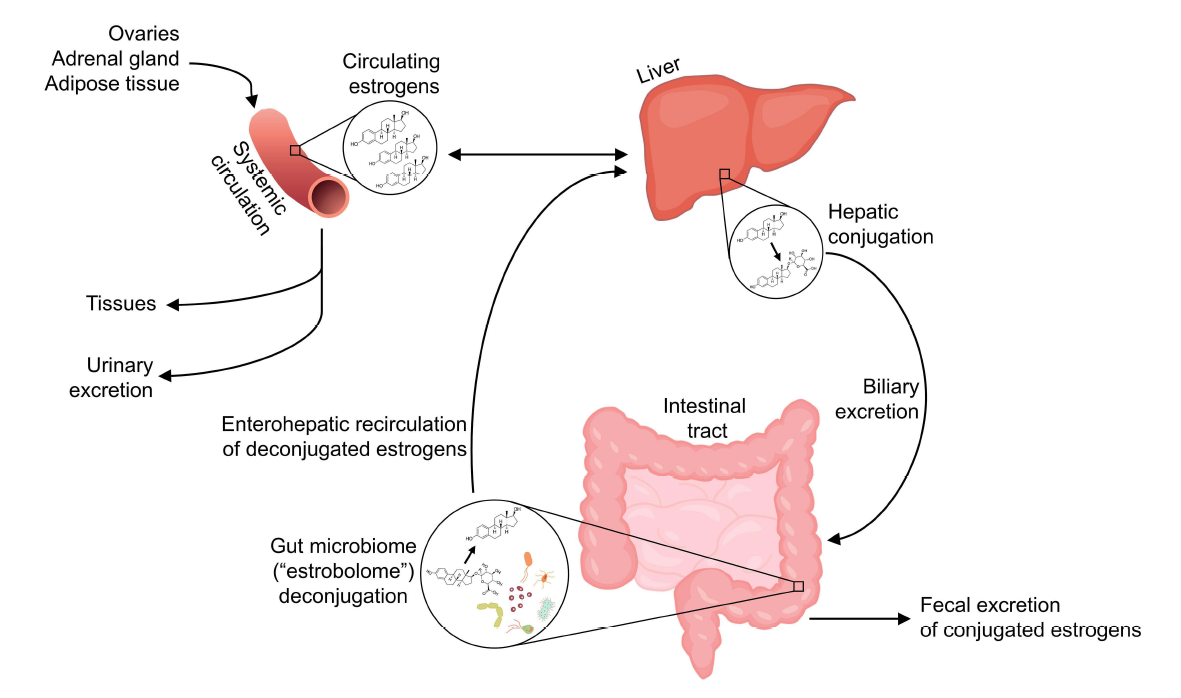
Exogenous phytoestrogens (soya isoflavones -SIF-, stilbene, coumestan, and lignans) are also metabolised by the oestrobolome in this manner, thereby enabling their ability to act via mechanisms involving ERα and ERβ in the same way, albeit with weaker binding capacity. Therefore, both oestrogens and phytoestrogens can act genomically by binding to oestrogen receptors leading to physiological changes across a variety of tissues previously described. Similarly, dysbiosis in the form of a reduction in gut microbiota diversity leads to a decrease in oestrogen metabolism, through a lack of oestrogen-metabolising bacteria which may influence hypoestrogenic-related symptoms.
Though much literature focuses on the gut microbiome and oestrogens, other hormones excreted in bile including progesterone and androgens are similarly deconjugated and recirculated by the gut microbiome. Thus, presumably, sex hormones influence the gut microbiota in part by serving as substrates for microbial metabolism, and in turn the gut microbiota influence sex hormone levels via deconjugation.
In summary, we can conclude that maintaining a healthy gut microbiome with a plant-based diet focusing on dietary fibre through vegetables, whole grains, nuts, seeds, berries, herbs, beans and pulses is recommended to support the gut microbiome, and therefore, the oestrobolome too. Consuming phyto-oestrogens, which are naturally abundant in soy as well as linseeds, wholegrains and pulses should be included to positively impact the oestrobolome.
Emerging research in mice shows that probiotic supplementation with various strains of Lactobacillus plantarum and Bifidobacterium longum in conjunction with soy isoflavones alters the metabolites of the gut microbiota that increases the circulating oestrogen level, upregulates the expression of oestrogen receptor α in abdominal adipose tissue and improves the production of SCFA’s, demonstrating the combination could attenuate the metabolic-related symptoms of menopause⁶.
The gut-brain axis
Postmenopausal women show a decline in cognition, most notably in memory, as a result of declining oestrogen levels, as well as depression and anxiety.
Gut bacteria can communicate with the brain via the gut-brain axis (GBA). Some metabolites produced by gut microbiota – Short-chain fatty acids (SCFAs), such as butyric acid, propionic acid and acetic acid, are able to stimulate the sympathetic nervous system, mucosal serotonin release and influence memory and learning process⁵. In this context, it is interesting to report that diet manipulation of microbiota may influence behaviour and mood.
Microbiota also interact with the gut-brain axis through different mechanisms, the principal one likely being the modulation of the intestinal barrier. Probiotic species-specific central effects are indeed associated with the restoration of tight-junction integrity and the protection of the intestinal barrier. Pre-intervention of animals with a probiotic combined formulation of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 restored tight junction barrier integrity and attenuated HPA axis and autonomic nervous system activities, assessed through plasma cortisol and catecholamine measurements.
A study of 54 women with vasomotor menopausal symptoms displayed a pronounced imbalance of the microbiota, which was characterized by a significant decrease in the main representatives of the obligate microflora (Bifidobacterium, Lactobacillus) and an increase in the number of opportunistic strains (Klebsiella and Clostridiodes difficile). In this picture, there was a disorder in the transmission of sensory information from the intestine to the central structures of the brain (cerebral cortex) due to altered expression of the mucous membrane receptors, hypercatecholaminemia, and activation of the sympathetic part of the autonomic nervous system. This leads to increased secretion of biological substances such as serotonin, histamine, and kinins, which in turn cause “neuro-endocrine-mediator chaos” that directly relates to vasomotor symptoms such as hot flushes, sleep disturbances and anxiety¹⁵.
Thus, potential imbalances in the gut microbiota composition during and after menopause might impact the mental health of women. Moreover, enteric microorganisms via the oestrobolome activity might also contribute to the alleviation of vasomotor symptoms such as hot flushes in perimenopausal women, when reactivated and reabsorbed oestrogen is transported to the brain. Reciprocally, neuropsychiatric conditions, such as stress or anxiety, can influence gut epithelial integrity and gut microbiota composition via the cascade of endocrine and neuroimmune signalling, involving the release of cortisol, again demonstrating the bidirectional relationship of all pathways.
Conclusions
In summary, the research to date on menopause, female sex hormones, and the gut microbiome suggests that menopause and/or low oestrogens are associated with reduced gut microbiome diversity and oestrobolome potential. Additionally, declines in oestradiol and progesterone may lead to permeability of the gut barrier, allowing microbial translocation to occur. Given the potential to modify the gut microbiome through specific prebiotic, probiotic, diet and lifestyle interventions, the gut microbiome represents an exciting frontier in peri- and post-menopausal health.
About Tanya Borowski
Tanya Borowski has spent the past two decades studying nutrition, human biology, functional medicine & women’s health education. She has empowered thousands with her workshops, retreats, 1:1 clinical work and practitioner programmes. Tanya uses a whole-person approach – that involves detecting underlying interferences that can inhibit the body’s ability to function or indeed heal.
As a registered nutritionist & Institute for Functional Medicine certified practitioner she is dedicated to helping women reduce their risk for chronic disease and ill health. She works to educate and empower women across all aspects of their life stages, from menarche to menopause to achieve their personal optimal health and well-being.
This article was written by guest blogger Tanya Borowski. If you are interested in writing an article for us, please contact us at info@invivohealthcare.com.